| Faculty Profile |
|
Office Address
540 East Canfield Avenue
Detroit, MI 48201
Office Phone
313-577-0475
Biography
Dr. Lash’s research program over the past nearly four decades has focused on various aspects of determining how chemicals produce injury to the kidneys and how we can design approaches to preventing or correcting such injury. The experimental models have included in vivo studies in rats and mice and in vitro studies using established cell lines and freshly isolated tissue and primary cell culture from rodent and human kidney. The chemicals used to produce kidney toxicity fall into one of three categories: 1) Model chemicals that are used to study specific mechanisms of action; 2) pharmacologic agents whose clinical use is dose-limited by nephrotoxicity, such as analgesics, antibiotics, and antiviral agents; and 3) environmental chemicals, such as inorganic mercury and tri- and perchloroethylene. We have also explored the impact of chronic diseases (e.g., diabetes) and pathological states (e.g., reduced nephron mass with compensatory renal hypertrophy or hypoxia) on susceptibility of the kidneys to chemically induced toxicity.
 |  |
Key research accomplishments:
- Discovery and characterization of a sodium-coupled GSH transporter on the renal basolateral membrane that accounts for most of the clearance of GSH in the renal circulation.
- Identification of mitochondria as a major subcellular site of action for nephrotoxic cysteine S-conjugates of halogenated solvents, such as trichloroethylene, in rat and human proximal tubular cells.
- Development of in vitro procedures to isolate cell suspensions from proximal and distal tubular regions of the rat nephron, to enable investigation of factors that determine cell type specificity of susceptibility to pathological and chemically induced injury.
- Characterization of sex- and species-dependent differences in metabolism and sensitivity to trichloroethylene and perchloroethylene, providing data for human health risk assessment.
- Identification of the dicarboxylate carrier (DIC; Slc25a10) and the oxoglutarate carrier (OGC; Slc25a11) of the mitochondrial inner membrane as being responsible for transport of cytoplasmic GSH into renal and hepatic mitochondria.
- Characterized protein expression and transport function of a battery of organic anion, organic cation, and amino acid transporters and expression and function of Phase I and Phase II drug metabolism enzymes in primary cultures of human proximal tubular cells, validating these cells for use in drug development and toxicity studies.
- Demonstrated adaptive changes in mitochondrial GSH transport and redox status in proximal tubular cells from kidney of diabetic or uninephrectomized rats.
 |
- Screened novel polymyxin antibiotic derivatives in primary cultures of human proximal tubular cells and in vivo in rats to identify those that maintain their antibacterial efficacy but exhibit less adverse effects (i.e., nephrotoxicity).
Current Research Directions
Mitochondrial Biomarkers of Renal Injury from Environmental and Therapeutic Agents
Exposure to a broad range of environmental contaminants, including halogenated solvents, polycyclic aromatic hydrocarbons, and heavy metals, can cause kidney injury. Acute kidney injury (AKI) is also a dose-limiting side effect of several classes of therapeutic drugs, including many antibiotics, antiviral agents, anticancer chemotherapeutic agents, and NSAIDs. Although some highly sensitive protein biomarkers have been validated in recent years, they are still associated with some degree of renal damage. Markers that can indicate exposure yet be detected prior to any or after only minimal injury are preferable. Additionally, identification of new markers that are more closely linked to mechanism of action will enhance understanding of mechanism and improve therapeutics. Our approach to identification of such mechanistic markers has focused primarily on the mitochondria as common, early, and sensitive targets in proximal tubular cells for an array of environmental contaminants and therapeutic drugs.
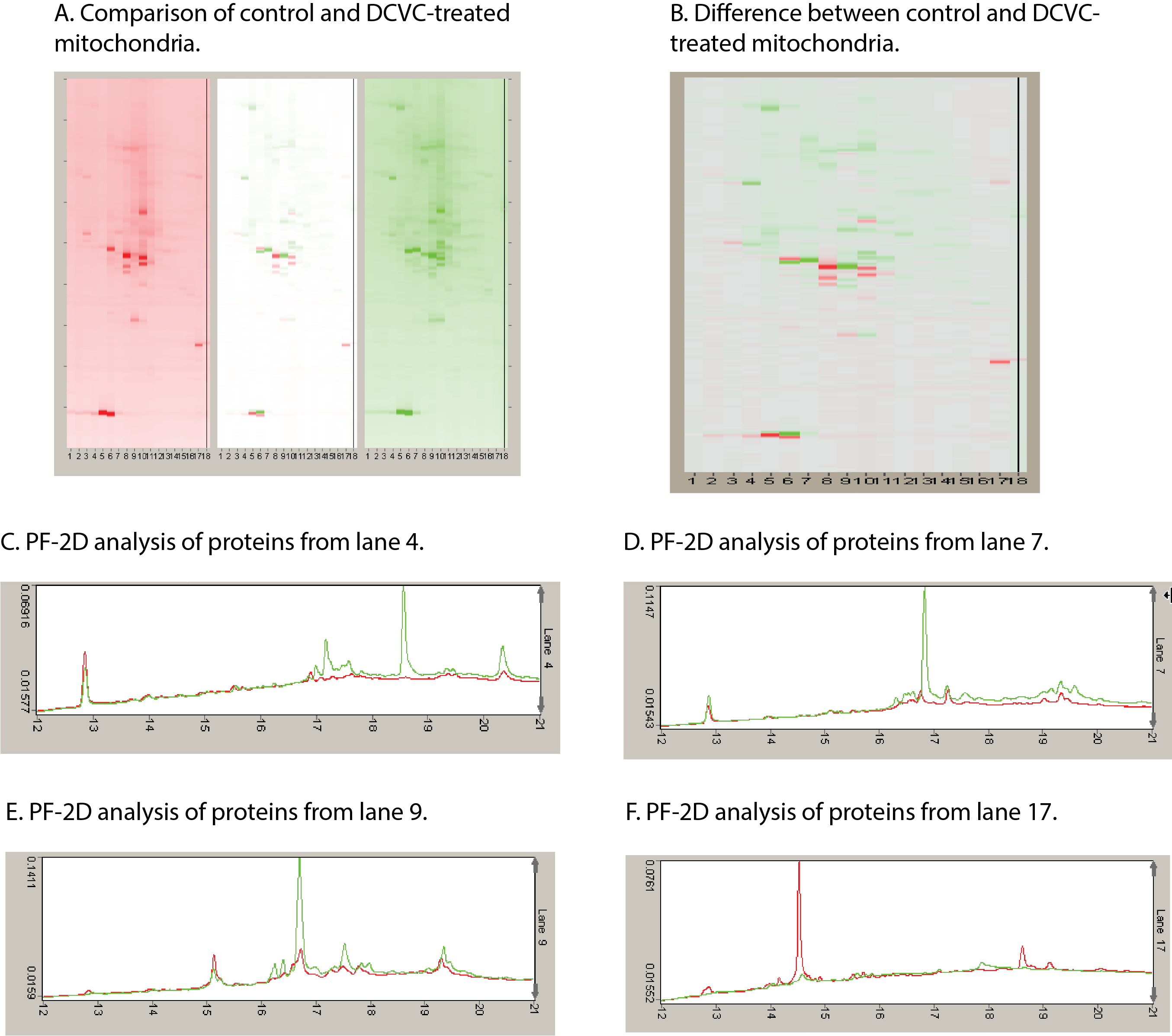 | 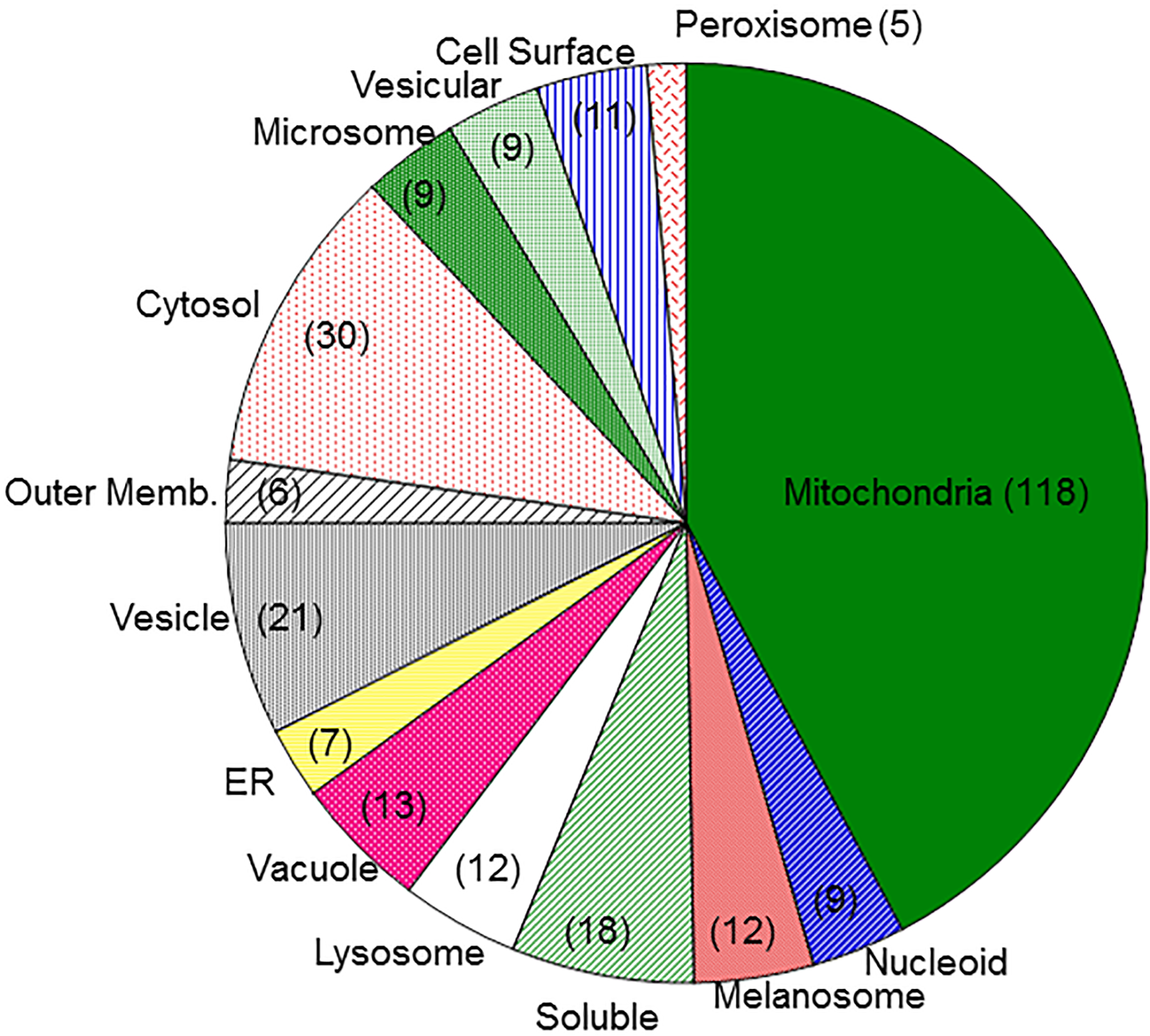 |
Using primary cultures of proximal tubular cells from human kidneys (hPT cells), the overall hypothesis being tested is that exposure of hPT cells to environmentally or therapeutically relevant concentrations of toxicants or therapeutic agents, respectively, will modify mitochondria and other cellular components and result in release of selected proteins and lipids and altered patterns of metabolites. hPT cells are treated with two environmental contaminants (the trichloroethylene metabolite S-(1,2-dichlorovinyl)-L-cysteine [DCVC] and HgCl2) or three therapeutic agents (tenofovir disoproxil fumarate, cisplatin, and polymyxin B) that all target renal mitochondria. Preliminary studies have helped refine the hypothesis and identified three specific proteins, one mitochondrial (sulfite oxidase), one cytoskeletal (keratins), and one cytoplasmic (HSP90), as potential biomarkers and now allow us to take a targeted approach for validation.
Targeted proteomics is being used to further validate release of proteins as well as to identify modified proteins that are associated with chemical exposures. Lipidomics and metabolomics are being used to identify release of cardiolipins and other lipids and differences in patterns of intermediary metabolites associated with chemical exposures. These releases and modifications are being correlated with renal cell function and viability as well as other well-established biomarkers. Another series of novel studies are exploring the potential identification of proteins in exosomes, as another potential source of sensitive markers of exposure and injury.
Office Fax
313-577-6739
Education
Baccalaureate: Case Western Reserve University, Cleveland, Ohio
Degree: B.A., Biology and French; granted May, 1980
Dates of Attendance: August, 1976 to May, 1980
Graduate: Emory University, Atlanta, Georgia
Degree: Ph.D., Biochemistry; granted May, 1985
Dates of Attendance: September, 1980 to December, 1984
Dissertation: Functions of the basal-lateral membrane in thiol metabolism
Ph.D. Advisor: Dr. Dean P. Jones
Fellowships
University of Rochester, Rochester, New York
Postdoctoral Fellow, Department of Pharmacology and Division of Toxicology, Department of Radiation Biology and Biophysics
Dates of Appointment: January, 1985 to October, 1986
Postdoctoral Advisor: Dr. M.W. Anders
Prior Appointments
University of Rochester, Rochester, New York
Instructor, Department of Pharmacology
Dates of Appointment: October, 1986 to February, 1988
Wayne State University, Detroit, Michigan
Assistant Professor, Department of Pharmacology
Dates of Appointment: March, 1988 to August, 1994
Wayne State University, Detroit, Michigan
Member, NIEHS Center for Molecular Toxicology with Human Applications
Dates of Appointment: August, 1989 to March, 2010
Wayne State University, Detroit, Michigan
Associate Professor, Department of Pharmacology
Dates of Appointment: August, 1994 to August, 2001
Wayne State University, Detroit, Michigan
Associate Professor with tenure, Department of Pharmacology
Dates of Appointment: August, 1995 to August, 2001
Marshall University, Huntington, WV
Associate Member (DS), Graduate Faculty
Dates of Appointment: August 1, 1999 to September 30, 2006
Professional Experience
- Consultant to National Research Council, U.S. Environmental Protection Agency, and National Toxicology Program for several human health risk assessments.
- Member, U.S. EPA Children’s Health Protection Advisory Committee (2012 – 2017).
- Workshop participant for multiple monographs for the International Agency for Research on Cancer (IARC).
- Member, U.S. EPA Science Advisory Board’s Chemical Assessment Advisory Committee (2012 – 2018); ad hoc 2019, 2020.
- Study section member and ad hoc participant: NIH, CDC/NIOSH.
- Founding Editor-in-Chief, Toxicology Reports (2013 – 2017, 2022 – present)
- Editor-in-Chief, Toxicology and Applied Pharmacology (2016 – present)
- Associate Editor, Journal of Pharmacology and Experimental Therapeutics (2004 – 2022)
- Associate Editor, Pharmacology & Therapeutics (2004 – 2024)
Publications
L.H. Lash. Unexpected enhancement of cytotoxicity of cisplatin in a rat kidney proximal tubular cell line overexpressing mitochondrial glutathione transport activity. Int. J. Mol. Sci. 23(4), 1993 (2022). https://doi.org/10.3390/ijms23041993 [PMID: 35216119]
L.H. Lash. Cellular biomarkers of renal injury. Curr. Opin. Toxicology 31, 100348. https://doi.org/10.1016/j.cotox.2022.100348 (2022).
L.H. Lash, P.M. Stemmer: Renal Mitochondria as Sentinels for Exposures to Environmental Toxicants and Nephrotoxic Drugs. In Toxicological Risk Assessment and Multi-System Health Impacts from Exposure, (A. Tsatsakis, ed.), Chapter 16, pp. 175-187, Amsterdam: Elsevier (2021). ISBN: 978-0-323-85215-9.
L.H. Lash. Diverse Roles of Mitochondria in Renal Injury from Environmental Toxicants and Therapeutic Drugs. Int. J. Mol. Sci. 22, 4172. https://doi.org/10.3390/ijms22084172 (2021). [PMID: 33920653]
L.H. Lash. Environmental and genetic factors influencing kidney toxicity. Semin. Nephrol. 39, 132-140 (2019). [PMID: 30827336]
Faculty Status
Cell Biology, Toxicology
